Parallel Amplified Saliva rapid POint-of-caRe Test (PASPORT™)

“Our invention ticks all the boxes for an ideal rapid test:
- ease of collection of saliva;
- highly accurate with very low false negative results, making it an invaluable screening tool;
- and can be done at any time of the day,
- making it possible to be used at point of care, with reliable authentication.
With this, we hope that more people will do the test as a personal act of social responsibility before engaging, especially, in large-scale events or gatherings.” – Professor Soo Khee Chee, Benjamin Sheares Professor in Academic Medicine at the SingHealth Duke-NUS Oncology Academic Clinical Programme, a Senior Advisor to Duke-NUS, and a senior co-inventor of the PASPORT™ Gold technology.
The PASPORT™ Gold technology is developed by a team of infectious diseases and engineering professors in Singapore, and has been validated in a clinical study at the Singapore General Hospital.
The PASPORT™ Gold COVID-19 Saliva Antigen Rapid Test (PASPORT™ Gold) is a single-use, highly sensitive test kit intended to detect the novel coronavirus SARS-CoV-2 that causes COVID-19 from saliva/drool. This test is designed for professional use with self-collected saliva/drool samples in individuals who are suspected of COVID-19.
PASPORT™ Gold is the first COVID-19 antigen rapid test (ART) with a signal amplification step that enhances the detection signal for increased sensitivity. As such, PASPORT™ Gold addresses key limitations with existing saliva-based ART kits (requirement for deep throat coughing, fasting), and can be performed at all times during the day.
Beyond COVID-19, Digital Life Line has developed the signal amplification platform to be easily adapted into existing ARTs to enhance detection sensitivity. Requiring minimal development work and no change to existing manufacturing workflow, this will enable existing ART manufacturers to achieve the same level of superior detection sensitivity in other infectious diseases, cardiac conditions, drug testing, and cancer.
Product Specification
|
Principle |
Chromatographic Immunoassay |
|
Format |
Cassette |
|
Specimen |
Saliva Sample |
|
Reading Time |
15-30 minutes |
|
Storage Temperature |
2-30°C |
|
Sensitivity (from pilot study) |
97% |
|
Specificity (from pilot study) |
90.6% |
* Tng, Danny Jian Hang, et al. “Amplified parallel antigen rapid test for point-of-care salivary detection of SARS-CoV-2 with improved sensitivity.” Microchimica Acta 189.1 (2022): 1-12.
Parallel Amplified Saliva rapid POint-of-caRe Test (PASPORT™)
Inventors: Dr Danny Tng (SGH/Duke-NUS), Professor Zhang Yong (NUS Biomedical Engineering), Associate Professor Melvin Chua (NCCS/Duke-NUS), Associate Professor Jenny Low (SGH/Duke-NUS), Professor Ooi Eng Eong (Duke-NUS), Professor Soo Khee Chee (NCCS/Duke- NUS)
In the ongoing COVID-19 pandemic, simple, rapid, point-of-care tests not requiring trained personnel for primary care testing are essential. Saliva-based Antigen Rapid Tests (ARTs) can fill this need, but these tests require overnight-fasted samples; without which, independent studies have demonstrated sensitivities of only 11.7 to 23.1%.
PASPORT™ is unique in that it adds a second type of nanoparticle that binds the first set of virus-binding nanoparticles to amplify the signal. In addition, the virus was captured using two distinct mechanisms at the test (T) line, using antibodies against the virus and proteins that the SARS-CoV-2 virus binds to. This makes testing using PASPORT™ more sensitive at finding and flagging the virus, and allows it to be used at any time of the day—its sensitivity is not compromised even after eating or drinking. Compared to other amplification techniques, PASPORT™ is able achieve detection even at much lower viral loads, enabling it to be extremely sensitive. In a clinical study involving over 100 participants conducted at the Singapore General Hospital, PASPORT™’s sensitivity in detecting different SARS-CoV-2 variants was 97 per cent and its specificity, 90.6 per cent, compared to the gold standard PCR test.
PASPORT™ has the potential to be developed for point-of-care testing, which may be particularly important in resource limited settings and for early diagnosis to initiate newly approved therapies to reduce COVID-19 severity.
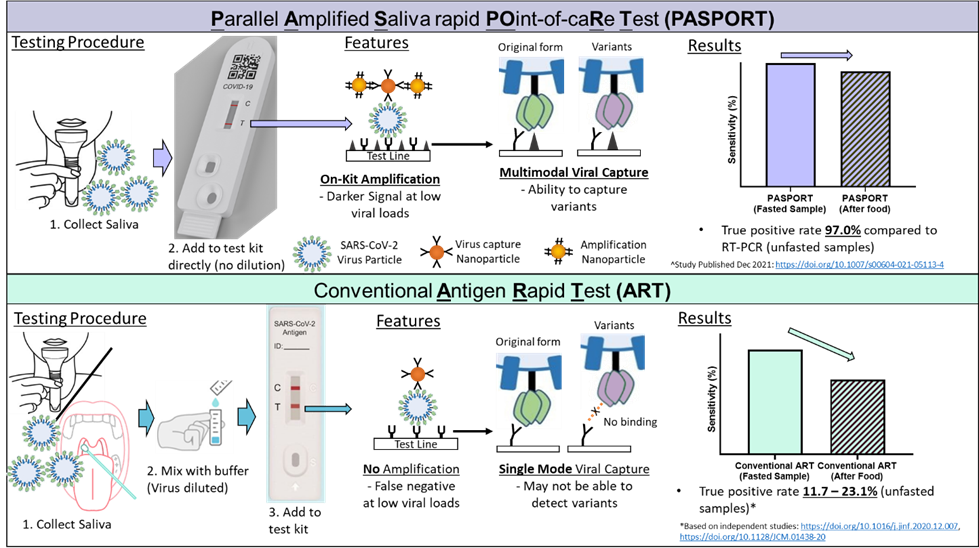
Schematic working principle of the PASPORT™ compared against a conventional antigen rapid test.
