About Us
Incorporated in 2021, Digital Life Line is a Singapore-based ISO13485-certified medical technology company that combines clinical expertise, global manufacturing know-how, and regulatory proficiency to rapidly bring to market diagnostics that enables accurate and convenient early screening.
DLL has a proven track record of working with renowned institutions in Singapore to accelerate the commercialization of best-in-class, highly de-risked breakthroughs through manufacturing, regulatory and market distribution.
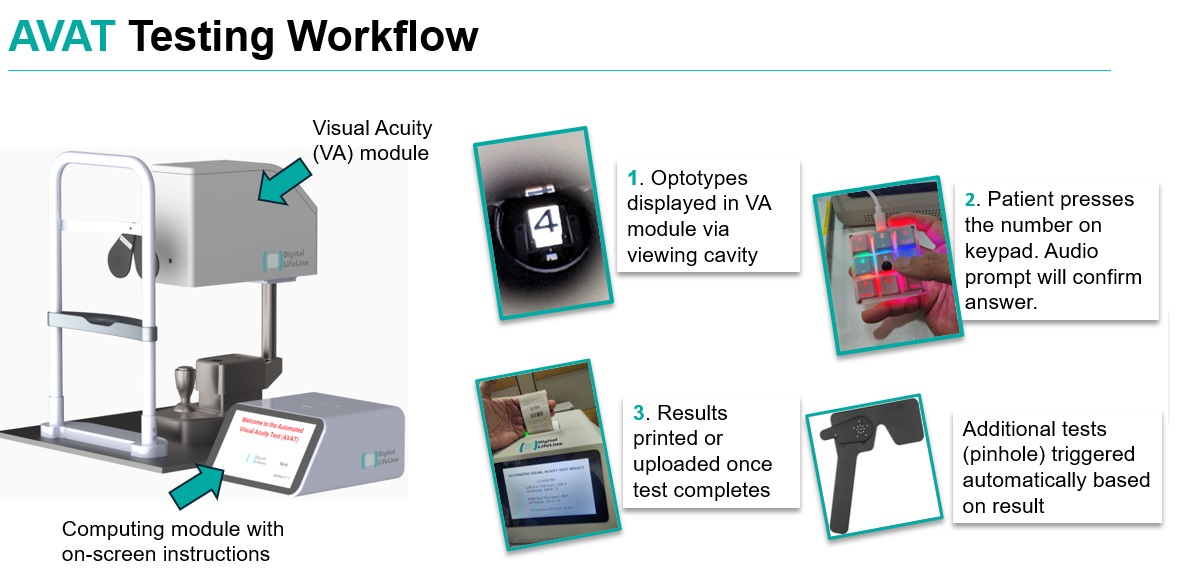
AVAT is an self-automated visual acuity testing device that addresses the needs across public hospitals for fast and accurate screening measurements with smaller space occupancy.
AVAT (Automated Visual Acuity Testing) aims to decentralize eye screening and disease monitoring, meeting the rising demand for accessible care. By automating visual acuity tests, AVAT efficiently scales up testing, minimizes costs, and optimizes manpower resources. The device addresses current challenges by providing a streamlined, cost-effective, and accessible solution for widespread visual acuity testing.

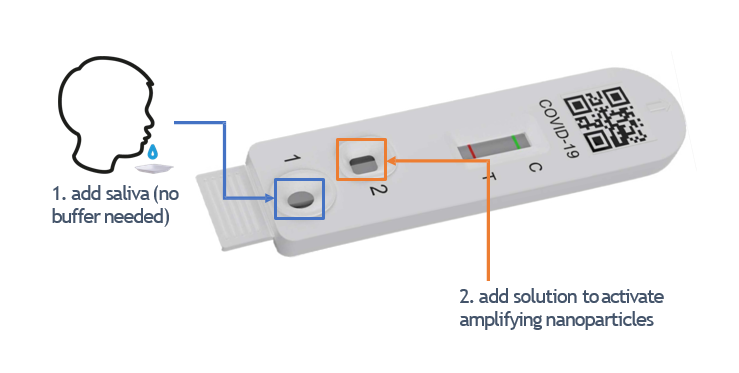
DLL has licensed the saliva-based ART kit IP from Duke-NUS Medical School and SingHealth. The kit, known as Parallel Amplified Saliva rapid POint-of-caRe Test (PASPORT), uses nanoparticles to bind the virus and uniquely, it also adds as a second type of nanoparticle that binds the first set of nanoparticles to amplify the signal. This makes testing using PASPORT more sensitive at finding and flagging the virus and allows it to be used at any time of the day, where the sensitivity of the test is not compromised even after eating or drinking. Compared to other ART, amplification enables PASPORT to be extremely sensitive. In a clinical study involving over 100 participants conducted at SGH, PASPORT’s sensitivity in detecting the virus was 97% and its specificity at 90.6%, compared to the gold standard PCR test. The invention was reviewed and published by Microchimica Acta* in November 2021.
*Microchimica Acta, established in 1937 by Fritz Pregl, is a monthly peer-reviewed scientific journal published by Springer Nature.

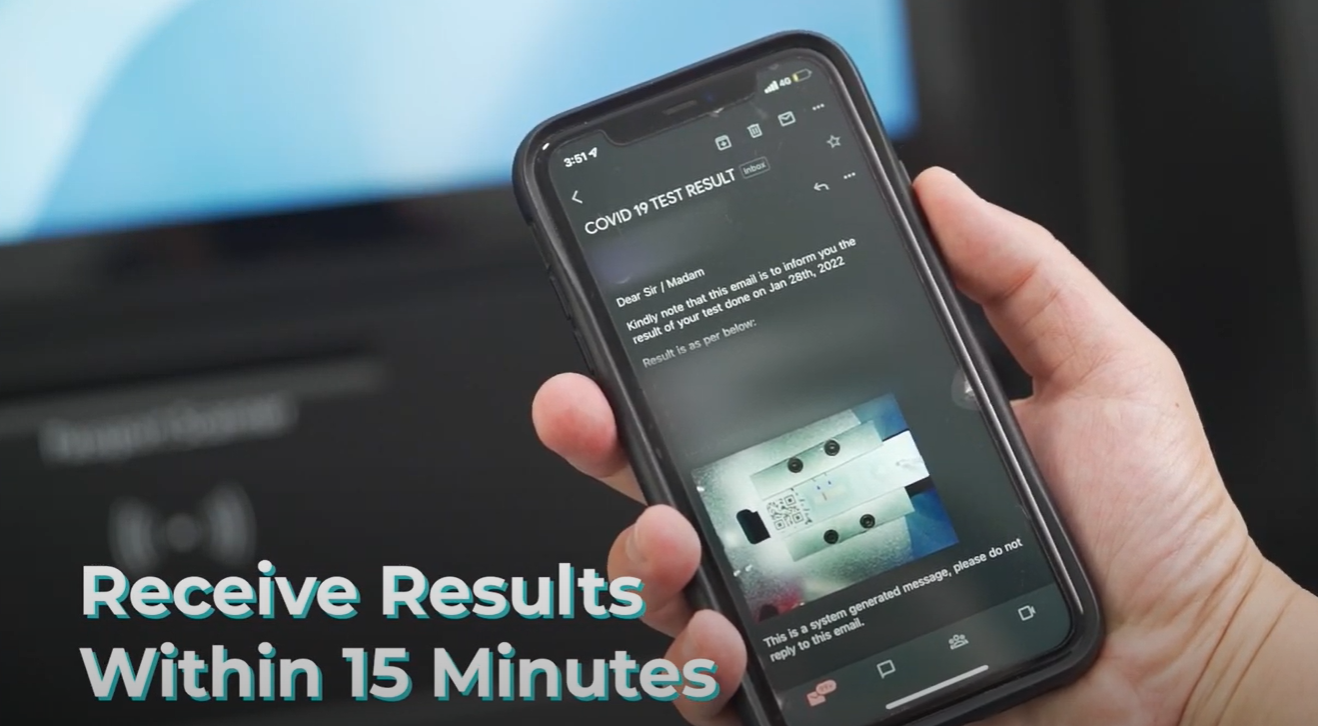
Our Automated Recording Antigen Rapid Testing (ART) Machine (“ChekPoint”)
Leveraging on DiSa Serialization technology, the Company has developed, an automated recording ART (Antigen Rapid Test) Machine (“ChekPoint”) that is capable of reading the results of ART cassette and accordingly, authenticate and automate the delivery of tampered-free test results to the intended recipients or authorities.