News and Media
Digital Life Line signs licensing agreement with NUH and NUS on next-generation automated vision screening solutions
Following the successful partnership on the PASPORT signal amplification platform with Duke-NUS and SingHealth, Digital Life Line has executed licensing agreements with National University Hospital Singapore (NUH) and National University of Singapore (NUS) to manufacture and deploy vision screening innovations into the community.
By incorporating AI algorithms, Automated Visual Acuity Test device (“AVAT”) and Smart, User-friendly Portable Reliable Automated perimetry device (“SUPRA”) represent an emerging trend of devices that can allow visual testing to be self-administered or with minimal help from a trained operator.
Read more in our press release
Follow us on LinkedIn ![]() and stay updated of our developments!
and stay updated of our developments!
#visionscreening #commercialisation
Pop the Champagne, We just turned ONE!🍾
2022 has been a fruitful year for Digital Life Line. (Scroll down ⬇️)
Milestones after milestone, we hit them all! We are thankful for the mentors, investors and industry partners that have worked along with us in our first year journey. 🤝








A toast to our journey! 🥂
Follow us on LinkedIn ![]() and keep updated of our next steps!
and keep updated of our next steps!
#wejustturnedONE #rapidantigentest #labqualityresult #PointofCareTesting
#rapiddiagnostic #medicaldevice #invitrodiagnostics
#healthcare #ecosystembuilding #mentorship
Digital Life Line Awarded International Medical Device Quality Certification 
We are proud to announce that we have achieved certification to ISO 13485:2016 and EN ISO 13485:2016, the quality management system standard for medical devices. This milestone, awarded by the British Standards Institution (“BSI”), demonstrates our capabilities in upholding global standards for medical device quality assurance. With these certifications, Digital Life Line (“DLL”) is one of the few Singapore medtech companies recognised for its adherence to global standards in the design, development, manufacture, import, storage and distribution of in- vitro diagnostic immunochromatographic test kits.
At DLL, we value innovation and are committed to developing high quality in-vitro diagnostic products with lab quality sensitivity to address global needs. Our core strength in innovation is drives our competency to design and develop medical devices in compliant with regulatory requirements set by the European Union (IVDD 98/79/EC) and EN ISO 13485:2016. From our Singapore HQ, we manage an extensive global supply chain network from manufacturing, import, storage and distribution to meet the diverse needs of our customers.
To learn more about DLL’s certifications and our platform to enhance sensitivity for rapid tests, visit and follow us on LinkedIn.
#medicaldevice #qms #iso13485 #rapiddiagnostics #pointofcare
Digital Life Line is at Medica, Düsseldorf and DxPx Conference!
November 16, 2022

3 completely packed days of meetings completed at Medica, Düsseldorf!
As part of the Scaler8 Germany mission trip organised by German Entrepreneurship Asia, Digital Life Line wasted no time and headed to the MEDICA – Leading International Trade Fair and DxPx Conference less than 6 hours after landing in Germany.

At the largest trade show for medical devices, Digital Life Line caught up with existing partners and engaged new industry connections as we set out on creating affordable rapid tests with lab quality sensitivity. We also heard from amazing pitches from 8 digital healthtech and diagnostics companies at the 42PLUS1 finals.
Following a productive 3 days, the team is now headed to Hamburg for more stakeholder meetings. If you are an innovative rapid test company looking to co-create best-in-class rapid tests for infectious diseases and others, feel free to drop us a note!
#rapidtest #labqualitysensitivity #invitrodiagnostics #antigentest #rapiddiagnostics
Digital Life Line Pte. Ltd., a Subsidiary of Disa Digital Safety Pte. Ltd. Secured New Fund From Investors and Its Existing Shareholders for an Aggregate Amount of Up to S$4 Million Based on a Pre–money Valuation of S$20 Million.
Appointment of Director and Establishment of Medical and Scientific Advisory Board at Digital Life Line Pte. Ltd., an Associate Company of DiSa Digital Safety Pte. Ltd
Digital Life Line Pte. Ltd., a subsidiary of DiSA Digital Safety Pte Ltd., received CE-IVD mark for its Saliva-based, Amplified SARS-CoV-2 Antigen Rapid Test (“ART”) Kit under the IVD Directive (98/79/EC)
New ‘ATM’ can validate self-swabbed ART for Covid-19
SINGAPORE – A local start-up here has come up with an “ATM” – not for withdrawing cash – but to supervise and validate one’s self-administered antigen rapid test (ART) for detecting Covid-19…
DISA: Advances to testing innovative Antigen Rapid Test kiosk
In Dec 2021, news broke about a breakthrough in the use of Antigen Rapid Test (ART) by top Singapore institutions — SingHealth Duke-NUS Academic Medical Centre and the National University of Singapore…
DiSa Digital Safety Pte. Ltd. signed proof of concept agreements with private healthcare service providers to test its automated antigen rapid test (“art”) machine
SINGAPORE – January 20, 2022 — Disa Digital Safety Pte. Ltd. has signed proof of concept agreements, through its 40% owned associate company, Digital Life Line Pte. Ltd. (“DLL”), with two large Singapore based private healthcare service providers to test the scalability of its patent pending Automated Antigen Rapid Test (“ART”) System (“Automated Machine”) in February 2022.

The Automated Machine requires users to self-register by scanning their NRIC or passport at the kiosk. Users are then required to scan the unique QR code on the ART kit on the Automated Machine to uniquely tag the ART kit to the user for track and trace purposes.
Users will perform the test in front of the Automated Machine in accordance to the test steps as illustrated on the screen of the Automated Machine. Users can then insert the ART cassette into the Automated Machine and leave without having to wait for the test result. The entire process is video recorded and takes no more than 5 minutes for each user. The Automated Machine will take a photo of the ART cassette and the results are sent automatically and digitally to the rightful owners within 15 to 20 minutes after the test is completed.
Each Automated Machine can handle about 10 to 15 tests per hour, operating for 24 hours a day. With the use of the Automated Machine, ART tests can be processed faster. Video recording guarantees the authenticity of the tests. Most importantly, all the ART test results cannot be tampered with as the ART cassette will have to be inserted into the Automated Machine before the result appears.
The Automated Machine hastens the entire ART process which enables service providers to provide more tests within a shorter period. The automation will also enable our partners to overcome the shortage of manpower. The digitalisation will also help to cope with the increasing ART tests volume in view of the fast spreading Omicron variant.
The Automated Machine can be deployed at dormitories, MICE, airports, train stations, hotels, schools, stadiums etc.
“We are pleased to test our technology in Singapore. Through partnerships with the healthcare service providers, we hope to expand this automation system regionally. The automated system can be used for any type of lateral flow point of care and not limited to only COVID-19”, said Mr Eddie Chng, the Managing Director and CEO of DLL.
DLL has licensed the saliva-based ART kit IP from Duke-NUS Medical School and SingHealth. The kit, known as Parallel Amplified Saliva rapid POint-of-caRe Test (PASPORT), uses nanoparticles to bind the virus and uniquely, it also adds as a second type of nanoparticle that binds the first set of nanoparticles to amplify the signal. This makes testing using PASPORT more sensitive at finding and flagging the virus and allows it to be used at any time of the day, where the sensitivity of the test is not compromised even after eating or drinking. Compared to other ART, amplification enables PASPORT to be extremely sensitive. In a clinical study involving over 100 participants conducted at SGH, PASPORT’s sensitivity in detecting the virus was 97% and its specificity at 90.6%, compared to the gold standard PCR test. The invention was reviewed and published by Microchimica Acta* in November 2021.

*Microchimica Acta, established in 1937 by Fritz Pregl, is a monthly peer-reviewed scientific journal published by Springer Nature.
About Digital Life Line Pte. Ltd.
DLL, a 40% owned associate company of Disa Digital Safety Pte. Ltd., is a Singapore-based company that deals with the manufacturing and distribution of saliva-based Antigen Rapid Test (ART) kits. DLL has signed tri-party licensing agreement with National University of Singapore (“NUS”) and Singapore Health Services Pte. Ltd. (“SingHealth”) on 8 December 2021 for the use of their new saliva-based COVID-19 ART technology. This technology was co-developed by SingHealth Duke-NUS Academic Medical Centre and the NUS.
About DiSa Digital Safety Pte. Ltd.
DiSa, a wholly-owned subsidiary of DISA Limited, is a Singapore-based technology solution provider that specializes in research and development of cutting-edge digital security solution (“DiSa Asset Protection System”). With its single scan technology and seamless integration, DiSa has been able to provide item level tracking and data with no disruption to the sales process. This technology is now protecting products and categories previously unachievable with traditional serialization methods, saving millions of dollars in prevented returns.
DiSa entered the US market in 2014 launching its Smart Solutions within the largest retailer in the world with a limited store test. After rigorous testing by the Loss Prevention Research Council, USA, DiSa rolled out its Smart Solutions nationwide in 2017.
About DISA Limited
DISA Limited (SGX: 532), is a publicly-traded company on the Singapore Catalist Stock Exchange. More information is available at http://www.digital-safety.com.
This announcement has been reviewed by the Company’s sponsor, SAC Capital Private Limited (“Sponsor”). This announcement has not been examined or approved by the Singapore Exchange Securities Trading Limited (“SGX-ST”) and the SGX-ST assumes no responsibility for the contents of this announcement including the correctness of any of the statements or opinions made or reports contained in this announcement.
The contact person for the Sponsor is Mr. Ong Hwee Li (Registered Professional, SAC Capital Private Limited)
Address: 1 Robinson Road, #21-00 AIA Tower, Singapore 048542.
Telephone number: +65 6232 3210
Saliva ART developed by S’pore scientists just as accurate as PCR tests, results in 15 minutes
December 10, 2021
SINGAPORE – Scientists here have developed a saliva antigen rapid test (ART) which is just as accurate as the polymerase chain reaction (PCR) test, yet takes only around 15 minutes to detect Covid-19…
DiSa partners NUS, SingHealth to commercialise new saliva-based Covid-19 ART kits
December 9, 2021
DIGITAL security firm DiSa $ DISA: 532 +50%, through its subsidiary company Digital Life Line, has signed a tripartite licensing agreement with the National University of Singapore (NUS) and Singapore Health Services (SingHealth) for the use of new saliva-based Covid-19 antigen rapid test (ART) kits….
Disa Digital Safety Pte. Ltd. signed licensing agreement with NUS and SingHealth to commercialise new saliva-based COVID-19 ART kits
SINGAPORE – December 9, 2021 — DiSa Digital Safety Pte. Ltd. (“DiSa”) has, through its subsidiary company, Digital Life Line Pte. Ltd. (“Digital Life Line”), signed a tri-party licensing agreement with National University of Singapore (“NUS”) and Singapore Health Services Pte. Ltd. (“SingHealth”) on 8 December 2021 for the use of their new saliva-based COVID-19 ART technology. This technology was co-developed by SingHealth Duke-NUS Academic Medical Centre and the NUS. Dubbed the Parallel Amplified Saliva rapid Point-of-caRe Test (PASPORT), the technology produces results in minutes, without the need for additional equipment or specially-trained personnel.
Despite the COVID-19 cases across the world remain high, many countries are re-opening their borders as they move over to the strategy of living with COVID-19. The demand for the ART kits is expected to increase as the number of the Vaccinated Travel Lane (VTL) arrangements between countries increases. Regular antigen rapid testing has become one of the preventive measures to contain the spread of the coronavirus. Workers from sectors such as construction, food and beverages, logistics, personal care services, etc. are mandated to perform antigen rapid testing on a regular basis. It is therefore important to ensure that the antigen rapid tests are conducted properly and the test results submitted are tampered-free.
In view of the ongoing COVID-19 pandemic, Digital Life Line plans to commercialise this new saliva-based ART kits, subject to Digital Life Line’s ability to obtain approval from the relevant regulatory authority, bundled with a system that is capable of reading the results of ART cassette and accordingly, authenticate and automate the delivery of tampered-free test results to the intended recipients or authorities. This system includes an automatic self-test kiosk and a professional-administered mobile application.
“Our system removes the administrative burden of the business owners who are required to do mandatory regular ART tests for their workers. We will be using DiSa Serialization technology to tag each unique user to an ART kit for track and trace purposes making it impossible for the user to tamper the test result. This technology has been used in US retail industry since 2017 to prevent theft and return fraud.” said Mr. Eddie Chng, Managing Director and Group Chief Executive Officer of DISA Limited.
About Digital Life Line Pte. Ltd.
Digital Life Line, a 93% owned subsidiary of Disa Digital Safety Pte. Ltd., is a Singapore-based company that deals with the manufacturing and distribution of saliva-based Antigen Rapid Test (ART) kits.
About DiSa Digital Safety Pte. Ltd.
DiSa, a wholly-owned subsidiary of DISA Limited, is a Singapore-based technology solution provider that specializes in research and development of cutting-edge digital security solution (“DiSa Asset Protection System”). With its single scan technology and seamless integration, DiSa has been able to provide item level tracking and data with no disruption to the sales process. This technology is now protecting products and categories previously unachievable with traditional serialization methods, saving millions of dollars in prevented returns.
DiSa entered the US market in 2014 launching its Smart Solutions within the largest retailer in the world with a limited store test. After rigorous testing by the Loss Prevention Research Council, USA, DiSa rolled out its Smart Solutions nationwide in 2017.
About DISA Limited
DISA Limited (SGX: 532), is a publicly-traded company on the Singapore Catalist Stock Exchange. More information is available at http://www.digital-safety.com.
This announcement has been reviewed by the Company’s sponsor, SAC Capital Private Limited (“Sponsor”). This announcement has not been examined or approved by the Singapore Exchange Securities Trading Limited (“SGX-ST”) and the SGX-ST assumes no responsibility for the contents of this announcement including the correctness of any of the statements or opinions made or reports contained in this announcement.
The contact person for the Sponsor is Mr. Ong Hwee Li (Registered Professional, SAC Capital Private Limited)
Address: 1 Robinson Road, #21-00 AIA Tower, Singapore 048542.
Telephone number: +65 6232 3210
SINGAPORE – January 20, 2022 — Disa Digital Safety Pte. Ltd. has signed proof of concept agreements, through its 40% owned associate company, Digital Life Line Pte. Ltd. (“DLL”), with two large Singapore based private healthcare service providers to test the scalability of its patent pending Automated Antigen Rapid Test (“ART”) System (“Automated Machine”) in February 2022.

The Automated Machine requires users to self-register by scanning their NRIC or passport at the kiosk. Users are then required to scan the unique QR code on the ART kit on the Automated Machine to uniquely tag the ART kit to the user for track and trace purposes.
Users will perform the test in front of the Automated Machine in accordance to the test steps as illustrated on the screen of the Automated Machine. Users can then insert the ART cassette into the Automated Machine and leave without having to wait for the test result. The entire process is video recorded and takes no more than 5 minutes for each user. The Automated Machine will take a photo of the ART cassette and the results are sent automatically and digitally to the rightful owners within 15 to 20 minutes after the test is completed.
Each Automated Machine can handle about 10 to 15 tests per hour, operating for 24 hours a day. With the use of the Automated Machine, ART tests can be processed faster. Video recording guarantees the authenticity of the tests. Most importantly, all the ART test results cannot be tampered with as the ART cassette will have to be inserted into the Automated Machine before the result appears.
The Automated Machine hastens the entire ART process which enables service providers to provide more tests within a shorter period. The automation will also enable our partners to overcome the shortage of manpower. The digitalisation will also help to cope with the increasing ART tests volume in view of the fast spreading Omicron variant.
The Automated Machine can be deployed at dormitories, MICE, airports, train stations, hotels, schools, stadiums etc.
“We are pleased to test our technology in Singapore. Through partnerships with the healthcare service providers, we hope to expand this automation system regionally. The automated system can be used for any type of lateral flow point of care and not limited to only COVID-19”, said Mr Eddie Chng, the Managing Director and CEO of DLL.
DLL has licensed the saliva-based ART kit IP from Duke-NUS Medical School and SingHealth. The kit, known as Parallel Amplified Saliva rapid POint-of-caRe Test (PASPORT), uses nanoparticles to bind the virus and uniquely, it also adds as a second type of nanoparticle that binds the first set of nanoparticles to amplify the signal. This makes testing using PASPORT more sensitive at finding and flagging the virus and allows it to be used at any time of the day, where the sensitivity of the test is not compromised even after eating or drinking. Compared to other ART, amplification enables PASPORT to be extremely sensitive. In a clinical study involving over 100 participants conducted at SGH, PASPORT’s sensitivity in detecting the virus was 97% and its specificity at 90.6%, compared to the gold standard PCR test. The invention was reviewed and published by Microchimica Acta* in November 2021.

*Microchimica Acta, established in 1937 by Fritz Pregl, is a monthly peer-reviewed scientific journal published by Springer Nature.
About Digital Life Line Pte. Ltd.
DLL, a 40% owned associate company of Disa Digital Safety Pte. Ltd., is a Singapore-based company that deals with the manufacturing and distribution of saliva-based Antigen Rapid Test (ART) kits. DLL has signed tri-party licensing agreement with National University of Singapore (“NUS”) and Singapore Health Services Pte. Ltd. (“SingHealth”) on 8 December 2021 for the use of their new saliva-based COVID-19 ART technology. This technology was co-developed by SingHealth Duke-NUS Academic Medical Centre and the NUS.
About DiSa Digital Safety Pte. Ltd.
DiSa, a wholly-owned subsidiary of DISA Limited, is a Singapore-based technology solution provider that specializes in research and development of cutting-edge digital security solution (“DiSa Asset Protection System”). With its single scan technology and seamless integration, DiSa has been able to provide item level tracking and data with no disruption to the sales process. This technology is now protecting products and categories previously unachievable with traditional serialization methods, saving millions of dollars in prevented returns.
DiSa entered the US market in 2014 launching its Smart Solutions within the largest retailer in the world with a limited store test. After rigorous testing by the Loss Prevention Research Council, USA, DiSa rolled out its Smart Solutions nationwide in 2017.
About DISA Limited
DISA Limited (SGX: 532), is a publicly-traded company on the Singapore Catalist Stock Exchange. More information is available at http://www.digital-safety.com.
This announcement has been reviewed by the Company’s sponsor, SAC Capital Private Limited (“Sponsor”). This announcement has not been examined or approved by the Singapore Exchange Securities Trading Limited (“SGX-ST”) and the SGX-ST assumes no responsibility for the contents of this announcement including the correctness of any of the statements or opinions made or reports contained in this announcement.
The contact person for the Sponsor is Mr. Ong Hwee Li (Registered Professional, SAC Capital Private Limited)
Address: 1 Robinson Road, #21-00 AIA Tower, Singapore 048542.
Telephone number: +65 6232 3210
SINGAPORE – Scientists here have developed a saliva antigen rapid test (ART) which is just as accurate as the polymerase chain reaction (PCR) test, yet takes only around 15 minutes to detect Covid-19.
The self-administered test has an accuracy rate of 97 per cent and is able to detect different Covid-19 viral variants, including Omicron.
The test kit may also be able to hit the market in as soon as three months.
The 15 minutes or so needed to obtain the results of the test, known as the Parallel Amplified Saliva rapid POint-of-caRe Test (Pasport), is similar to the shortest time needed for current ARTs. For PCR tests, it takes between a few hours and three days to get results.
The test is the result of a research collaboration between Duke-NUS Medical School, Singapore General Hospital (SGH), National Cancer Centre Singapore (NCCS) and the National University of Singapore (NUS).
Dr Danny Tng, a medical officer at the Department of Infectious Diseases in SGH, and the lead inventor behind the test, said that Duke-NUS and SingHealth have also entered into a licensing agreement with medical supply company Digital Life Line for its commercialisation.
Professor Soo Khee Chee, Benjamin Sheares Professor in Academic Medicine at the SingHealth Duke-NUS Oncology Academic Clinical Programme, said that the test, which requires the approval of the Health Sciences Authority for use here, could be available to consumers in the next three to six months.
One important innovation of the new test is that it can be done at any point in time – even after food. Current saliva tests have not been considered reliable enough to roll out on a large scale, as the concentration of viral particles in saliva “drops steeply” after one eats or drinks, Dr Tng noted.
For instance, the ability of other saliva ARTs to detect the Sars-CoV-2 virus after food is around 11.7 per cent to 23.1 per cent, he said.
“Therefore, saliva antigen rapid tests are usually reliable only when they are performed first thing in the morning, after an overnight fast and before breakfast or brushing teeth. This makes testing of saliva samples at other times of the day less reliable,” he added.
The scientists were able to remedy this using a two-stage process for the Pasport.
Like most ART, Pasport uses nanoparticles to bind to the virus, but with a difference – an additional amplification mechanism is built into it such that it uses more nanoparticles in its test than other ARTs, said Dr Tng.
This means the viral “signal” will be a lot stronger, allowing the Pasport to detect low viral loads, such as after a meal or drink, he added.
To capture viral variants which may otherwise evade detection through testing, the researchers have another trick up their sleeve.
Apart from using an antibody placed at the test line to capture viral proteins, just like in conventional ART kits, additional ACE2 proteins are used to capture the virus.
The ACE2 protein is the entry point for the coronavirus to infect human cells.
This is because viral variants may change their targeted protein structure, and may thus evade antibody detection, said Dr Tng.
Professor Ooi Eng Eong, from the Duke-NUS emerging infectious diseases programme, said: “As the virus evolves to become more infectious, like with Delta and Omicron, the evolution has to do with its ability to infect – and the key is through ACE2. So as the virus becomes more infectious, our test will work better.”
To validate these results, a clinical study involving more than 100 participants was conducted at SGH. The study demonstrated that Pasport’s sensitivity – the ability to identify those infected as positive – was at 97 per cent; while its specificity – the ability to correctly return a negative result – was at 90.6 per cent, compared to PCR tests.
PCR tests typically have a sensitivity rate of more than 99.5 per cent and a specificity rate of around 100 per cent. The World Health Organisation requires ARTs to have a sensitivity rate of above 80 per cent.
Prof Ooi said that with such a saliva test, general practitioners would be able to administer it for early diagnosis of Covid-19, without having to send a test sample to the lab for processing.
This could prevent people from developing severe Covid-19, he added, noting that many Covid-19 treatments have to be given in the early stages of infection for better results.
Prof Soo said the group’s invention has “ticked all the boxes” for an ideal rapid test.
The ease of collecting saliva also makes the test more convenient and preferable for those who find nasal swabs uncomfortable.
“With this, we hope that more people will do the test as a personal act of social responsibility before engaging, especially, in large-scale events or gatherings,” added Prof Soo.

DIGITAL security firm DiSa $ DISA: 532 +50%, through its subsidiary company Digital Life Line, has signed a tripartite licensing agreement with the National University of Singapore (NUS) and Singapore Health Services (SingHealth) for the use of new saliva-based Covid-19 antigen rapid test (ART) kits.
Digital Life Line plans to commercialise the ART kits, subject to its ability to obtain approval from the relevant regulatory authority. The subsidiary specialises in manufacturing and distributing the new saliva-based ART kits.
The kits will be bundled to a system capable of reading the results from the ART cassette, before authenticating and automating the delivery of the tamper-free test results to intended recipients. The system will also include an automatic self-test kiosk and a mobile application.
According to DiSa managing director and group chief executive officer Eddie Chng, the company’s serialisation technology will be used to tag each unique user to an ART kit, to prevent tampering of the test result.
DiSa highlighted the need for the ART kits to be tamper-free, considering the strong demand for these as Vaccinated Travel Lanes open up, and mandatory testing for workers from different sectors. Demand is expected to increase further as countries reopen and testing becomes more regular.
“Our system removes the administrative burden of the business owners who are required to do mandatory regular ART tests for their workers,” said Chng.
The saliva-based ART kits were developed by NUS and SingHealth Duke NUS Academic Medical Centre. On Wednesday (Dec 8), Duke-NUS Medical School announced that the new technology could test for Covid-19 “at the point-of-care with sensitivity higher than current ART tests and close to that of laboratory-based Polymerase Chain Reaction (PCR) tests”.
DiSa closed on Thursday (Dec 9) up by 0.2 Singapore cent or 100 per cent at 0.4 cent, after the announcement, with nearly 252.5 million shares traded.
SINGAPORE – December 9, 2021 — DiSa Digital Safety Pte. Ltd. (“DiSa”) has, through its subsidiary company, Digital Life Line Pte. Ltd. (“Digital Life Line”), signed a tri-party licensing
agreement with National University of Singapore (“NUS”) and Singapore Health Services Pte. Ltd. (“SingHealth”) on 8 December 2021 for the use of their new saliva-based COVID-19 ART
technology. This technology was co-developed by SingHealth Duke-NUS Academic Medical Centre and the NUS. Dubbed the Parallel Amplified Saliva rapid POint-of-caRe Test
(PASPORT), the technology produces results in minutes, without the need for additional equipment or specially-trained personnel.
Please refer to the Appendix 1 for more information about Duke-NUS’ invention, the first Singapore-invented ART technology that is hassle-free and fast in delivering the test result.
Despite the COVID-19 cases across the world remain high, many countries are re-opening their borders as they move over to the strategy of living with COVID-19. The demand for the ART kits
is expected to increase as the number of the Vaccinated Travel Lane (VTL) arrangements between countries increases. Regular antigen rapid testing has become one of the preventive
measures to contain the spread of the coronavirus. Workers from sectors such as construction, food and beverages, logistics, personal care services, etc. are mandated to perform antigen
rapid testing on a regular basis. It is therefore important to ensure that the antigen rapid tests are conducted properly and the test results submitted are tampered-free.
In view of the ongoing COVID-19 pandemic, Digital Life Line plans to commercialise this new saliva-based ART kits, subject to Digital Life Line’s ability to obtain approval from the relevant
regulatory authority, bundled with a system that is capable of reading the results of ART cassette and accordingly, authenticate and automate the delivery of tampered-free test results to the
intended recipients or authorities. This system includes an automatic self-test kiosk and a professional-administered mobile application.
“Our system removes the administrative burden of the business owners who are required to do mandatory regular ART tests for their workers. We will be using DiSa Serialization technology to tag each unique user to an ART kit for track and trace purposes making it impossible for the user to tamper the test result. This technology has been used in US retail industry since 2017 to prevent theft and return fraud.” said Mr. Eddie Chng, Managing Director and Group Chief Executive Officer of DISA Limited.
About Digital Life Line Pte. Ltd.
Digital Life Line, a 93% owned subsidiary of Disa Digital Safety Pte. Ltd., is a Singapore-based company that deals with the manufacturing and distribution of saliva-based Antigen Rapid Test (ART) kits.
About DiSa Digital Safety Pte. Ltd.
DiSa, a wholly-owned subsidiary of DISA Limited, is a Singapore-based technology solution provider that specializes in research and development of cutting-edge digital security solution (“DiSa Asset Protection System”). With its single scan technology and seamless integration, DiSa has been able to provide item level tracking and data with no disruption to the sales process. This technology is now protecting products and categories previously unachievable with traditional serialization methods, saving millions of dollars in prevented returns.
DiSa entered the US market in 2014 launching its Smart Solutions within the largest retailer in the world with a limited store test. After rigorous testing by the Loss Prevention Research Council, USA, DiSa rolled out its Smart Solutions nationwide in 2017.
About DISA Limited
DISA Limited (SGX: 532), is a publicly-traded company on the Singapore Catalist Stock Exchange. More information is available at http://www.digital-safety.com.
This announcement has been reviewed by the Company’s sponsor, SAC Capital Private Limited (“Sponsor”). This announcement has not been examined or approved by the Singapore Exchange Securities Trading Limited (“SGX-ST”) and the SGX-ST assumes no responsibility for the contents of this announcement including the correctness of any of the statements or opinions made or reports contained in this announcement.
The contact person for the Sponsor is Mr. Ong Hwee Li (Registered Professional, SAC Capital Private Limited)
Address: 1 Robinson Road, #21-00 AIA Tower, Singapore 048542.
Telephone number: +65 6232 3210
A new saliva-based COVID-19 Antigen Rapid Test (ART) technology co-developed by the SingHealth Duke-NUS Academic Medical Centre and the National University of Singapore shows promise in early clinical testing, outperforming existing ARTs and delivering results in minutes, with nearly comparable sensitivity to the gold standard Polymerase Chain Reaction (PCR) test.
 SINGAPORE, 8 December 2021 – A potentially game-changing Antigen Rapid Test (ART) technology to diagnose COVID-19 has been developed by scientists in Singapore. Using a proprietary on-kit amplification technique, a person’s saliva can be self-administered or tested for the SARS-CoV-2 virus at the point-of-care with sensitivity higher than current ART tests and close to that of laboratory-based polymerase chain reaction (PCR) tests.
SINGAPORE, 8 December 2021 – A potentially game-changing Antigen Rapid Test (ART) technology to diagnose COVID-19 has been developed by scientists in Singapore. Using a proprietary on-kit amplification technique, a person’s saliva can be self-administered or tested for the SARS-CoV-2 virus at the point-of-care with sensitivity higher than current ART tests and close to that of laboratory-based polymerase chain reaction (PCR) tests.
Dubbed the Parallel Amplified Saliva rapid POint-of-caRe Test (PASPORT), the technology produces results in minutes, without the need for additional equipment or specially-trained personnel. The invention was borne out of a research collaboration between Duke-NUS Medical School, Singapore General Hospital (SGH) and National Cancer Centre Singapore (NCCS)—collectively member institutes of the SingHealth Duke-NUS Academic Medical Centre—and the National University of Singapore (NUS).
With the impending availability of oral antiviral drugs against SARS-CoV-2, COVID-19 could potentially be diagnosed and treated in the primary care setting in the future. A test that can be done on-site will enable doctors to diagnose COVID-19 accurately and prescribe the drugs appropriately. Moreover, with its anticipated low cost and ease of use as compared to PCR tests, PASPORT could aid Singapore and countries around the world in making early diagnosis of COVID-19 to initiate appropriate case management. The research was published online on Monday, 6 December, 2021, in the journal Microchimica Acta as a feature article in their series, ‘From Bench to Hand’.
“Testing is an indispensable tool in the management of COVID-19 cases. Although PCR has been the gold standard, it requires trained personnel and laboratory infrastructure,” said lead inventor Dr Danny Jian Hang Tng, Medical Officer at the Department of Infectious Diseases, SGH, and an adjunct Research Fellow at Duke-NUS’ Emerging Infectious Diseases (EID) Programme. Dr Tng, who graduated from Duke-NUS in 2019, added, “A reliable and painless saliva antigen test that is affordable and convenient to perform would encourage more to be tested, and more frequent testing. This may enable us to manage COVID-19 more effectively not only at the point of care, but also in settings such as airports, conventions and even at home.”
Unlike tests that use nasal or throat swabs, saliva-based tests are convenient and are more easily self-administered. But, until now, saliva tests for detecting SARS-CoV-2 have not been considered reliable enough to roll out at large scale. This is because the concentration of viral particles in saliva drops steeply after an individual eats or drinks. As a result, saliva antigen tests are usually only reliable when they are performed first thing in the morning, after an overnight fast and before breakfast or brushing teeth. This makes testing of saliva samples at other times of the day less reliable.
The researchers remedied this by using a two-stage process. Like other ARTs, PASPORT uses nanoparticles to bind the virus. But uniquely, it also adds a second type of nanoparticle that binds the first set of nanoparticles to amplify the signal. This makes testing using PASPORT more sensitive at finding and flagging the virus, and allows it to be used at any time of the day—its sensitivity is not compromised even after eating or drinking. Compared to other amplification techniques, PASPORT is able achieve detection even at much lower viral loads, enabling it to be extremely sensitive. In a clinical study involving over 100 participants conducted at SGH, PASPORT’s sensitivity in detecting the virus was 97 per cent and its specificity, 90.6 per cent, compared to the gold standard PCR test.
Professor Ooi Eng Eong, from the Duke-NUS EID Programme, who is one of the senior co-inventors, said, “Like COVID-19 vaccines, the availability of oral anitiviral drugs will be another game changer in our fight against COVID-19. But these drugs will need to be given as early as possible after illness onset for maximal benefit. A test that can be self-administered or used on-site at the primary care setting may mitigate the need for cases to be managed at the hospitals.”
Professor Soo Khee Chee, Benjamin Sheares Professor in Academic Medicine at the SingHealth Duke-NUS Oncology Academic Clinical Programme, a Senior Advisor to Duke-NUS, and a senior co-inventor, said, “Our invention ticks all the boxes for an ideal rapid test: ease of collection of saliva; highly accurate with very low false negative results, making it an invaluable screening tool; and can be done at any time of the day, making it possible to be used at point of care, with reliable authentication. With this, we hope that more people will do the test as a personal act of social responsibility before engaging, especially, in large-scale events or gatherings.”
Duke-NUS and SingHealth have filed intellectual property protection for the invention, and have entered into a license agreement with Digital Life Line Pte Ltd, a Singapore-based company. The inventors hope that through close collaboration with commercial partners, the product can be out in the market as soon as possible to serve healthcare needs in Singapore and beyond.
*The full list of inventors are: Dr Danny Tng (SGH/Duke-NUS), Professor Zhang Yong (NUS), Associate Professor Melvin Chua (NCCS/Duke-NUS), Associate Professor Jenny Low (SGH/Duke-NUS), Professor Ooi Eng Eong (Duke-NUS), Professor Soo Khee Chee (NCCS/Duke-NUS)
The development of the PASPORT prototype was partially supported by the Estate of Tan Sri Khoo Teck Puat, of the Khoo Foundation, Singapore.
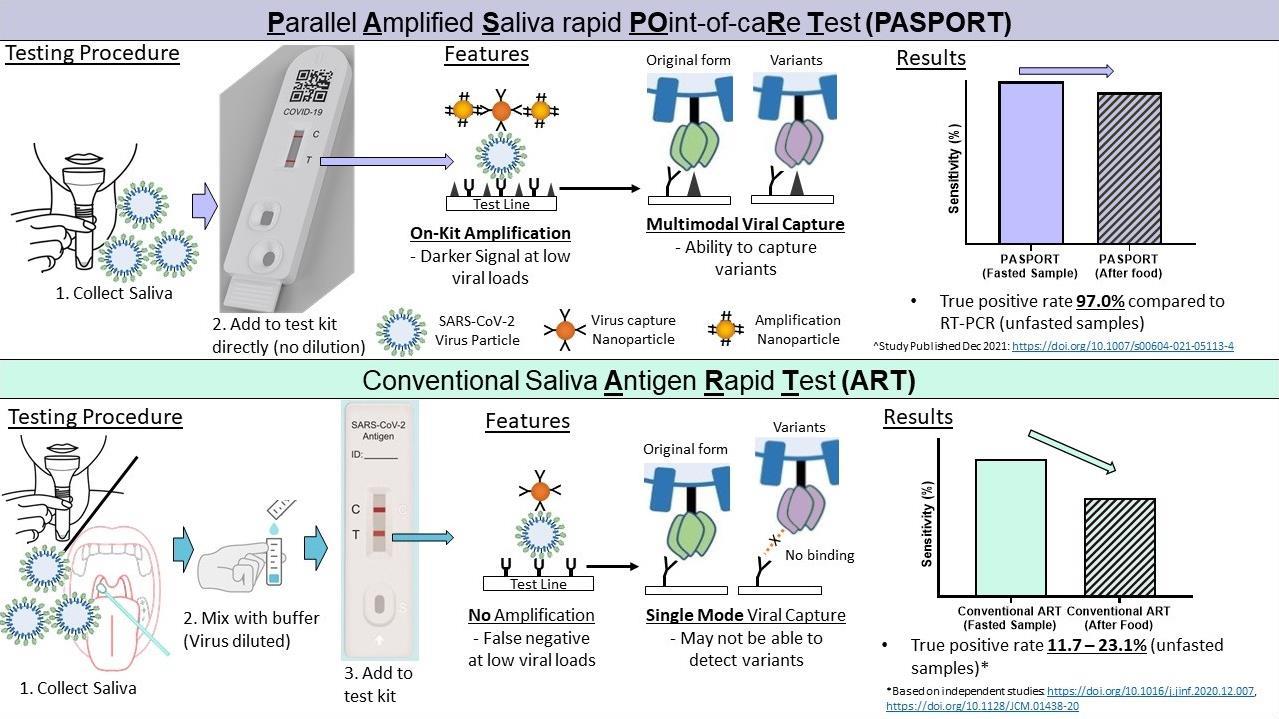
Comparison between how PASPORT and a conventional saliva-based ART processes samples.
For media enquires, pls contact:
- Federico Graciano, Duke-NUS Communications
- Lydia Ng, SingHealth Group Communications

