About Us
Incorporated in 2021, Digital Life Line is a Singapore-based ISO13485-certified medical technology company that combines clinical expertise, global manufacturing know-how, and regulatory proficiency to rapidly bring to market diagnostics that enables accurate and convenient early screening.
DLL has a proven track record of working with renowned institutions in Singapore to accelerate the commercialization of best-in-class, highly de-risked breakthroughs through manufacturing, regulatory and market distribution.
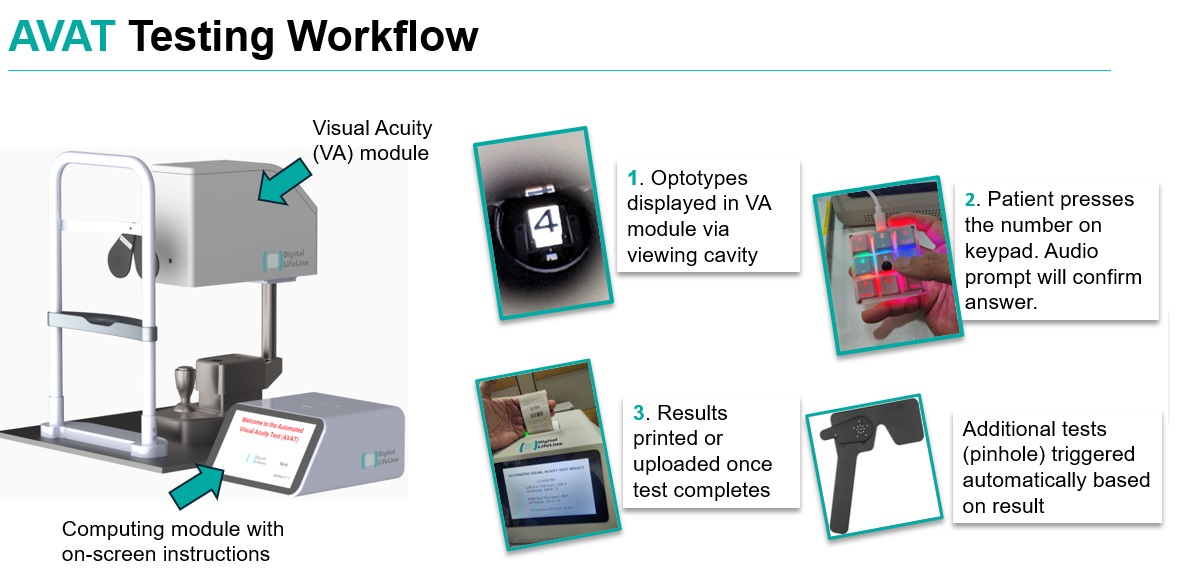
AVAT is an self-automated visual acuity testing device that addresses the needs across public hospitals for fast and accurate screening measurements with smaller space occupancy.
AVAT (Automated Visual Acuity Testing) aims to decentralize eye screening and disease monitoring, meeting the rising demand for accessible care. By automating visual acuity tests, AVAT efficiently scales up testing, minimizes costs, and optimizes manpower resources. The device addresses current challenges by providing a streamlined, cost-effective, and accessible solution for widespread visual acuity testing.